Sample Soil Test
We have combined world-class agronomic consulting with industry leading analytical tools to assess and diagnose your toughest turf issues. These solutions will help you manage your most valuable assets whether you manage a golf course, nursery, sod farm or sports fields.
Our Worldwide services include:
- Analytical testing of soils, waters and plant tissues.
- Custom recommendations based on experienced evaluation of your test results
- Historical and advanced trend charting of soil nutrition & irrigation suitability
- Advanced online tools for managing agronomic details
Soil Test
A soil test is a process by which elements (phosphorus, potassium, calcium, magnesium, sodium, sulfur, manganese, copper and zinc) are chemically removed from the soil and measured for their “plant available” content within the sample. A soil test also measures the quantity of available nutrients, soil pH, humic matter and exchangeable acidity. This analysis indicates whether lime is needed and if so, how much to apply.
SOIL pH – Desired Level 6 – (a measure of the acidity or alkalinity of the soil)
Soil pH is one of the most important soil properties that affects the availability of nutrients.
- Macronutrients – tend to be less available in soils with Low pH.
- Micronutrients – tend to be less available in soils with High pH.
Lime can be added to the soil to make it less sour (acid) and also supplies calcium and magnesium for plants to use. Lime can also raise the pH to the desired range of 6.0 to 6.5 in soils where needed. In this pH range, nutrients are more readily available to plants and microbial populations in the soil increase. Microbes convert nitrogen and sulfur to forms that are readily available for plant use. Lime also enhances the physical properties in the soil that promote water and air movement I adjusted properly.
NITROGEN (N)
Nitrogen is a part of all living cells and is a necessary part of all proteins, enzymes and metabolic processes involved in the synthesis and transfer of energy. Nitrogen is also a part of chlorophyll, the green pigment of the plant that is responsible for photosynthesis and helps plants with the following:
- Rapid growth and recovery
- Increasing seed and fruit production
- Improving the quality of leaf and forage crops
Nitrogen most often comes from fertilizer application and from the air (legumes get their N from the atmosphere, water or rainfall contributes very little Nitrogen)
PHOSPHORUS (P)
This is a measurement of the Phosphorous that is readily available in the soil. Like nitrogen, Phosphorous is an essential part of the process of photosynthesis. It is involved in the formation of all oils, sugars and starches. It helps with the following:
Transformation of solar energy into chemical energy, proper plant maturation and combatting environmental stresses
- Effects rapid growth
- Encourages blooming
- Sustains root growth
Phosphorus most often comes from fertilizer, bone meal and superphosphates.
POTASSIUM (K) – Desired Level .5 – 10 PPM
Potassium influences stress and wear tolerance and is absorbed by plants in larger amounts than any other mineral except nitrogen and in some cases, calcium. Potassium helps in the following:
- Building of proteins
- Photosynthesis
- Reduction of diseases
Potassium is supplied to plants by soil minerals, organic materials and fertilizer. Optimum levels change with the cation exchange capacity (CEC) in general; turf soils should be maintained at high levels.
CALCIUM (CA) – Desired Level 40 – 120 PPM
Calcium, an essential part of plant cell wall structure, provides for normal transport and retention of other elements as well as strengthening in the plant. It is also thought to counteract the effect of alkali salts and organic acids within a plant. Sources of Calcium are dolomitic lime, gypsum, and superphosphates.
MAGNESIUM (Mg) – Desired Level 6 – 24 PPM
Magnesium is part of the chlorophyll in all green plants and essential for photosynthesis. It also helps activate many plant enzymes needed for growth. Sources of Magnesium for plants are:
- Soil and Organic materials
- Fertilizers
- Dolomitic lime
SULFUR (S) (SO4) – Desired Level <200 PPM
Essential plant food for production of protein. It promotes activity and development of enzymes and vitamins and also helps in the formation of chlorophyll. It also improves root growth, seed production, helps with vigorous plant growth and protects the plant from extreme cold during winter. Sulfur may be supplied to the soil from rainwater pending geographical location. It is also added in some fertilizers as an impurity, especially by low grade fertilizers. The use of gypsum also increases soil sulfur levels.
BORON (B) – Desired Level .2 – .8 PPM
Helps with the consumption of nutrients and helps to regulate other nutrients while aiding in production of sugar and carbohydrates. It is essential for seed and fruit development. Sources of Boron are organic matter and borax.
COPPER (Cu)
Important for reproductive growth. Aids in root metabolism and helps in the utilization of proteins.
CHLORIDE (CI) – Desired Level <140
Aids plant metabolism. Chloride is found in the soil and can also come from treated and water run-off.
IRON (Fe) – Desired Level 2 – 5
Essential for formation of chlorophyll. Sources of Iron are the soil, Iron sulfate, Iron chelate.
MANGANESE (Mn)
Functions with enzyme systems involved in the breakdown of carbohydrates, nitrogen, and regulating metabolism. Soil is a source of manganese.
ZINC (Zn)
Is essential for the transformation of carbohydrates. It regulates consumption of sugars as part of the enzyme systems that regulate plant growth. Sources of Zinc are soil, Zinc oxide, Zinc sulfate and Zinc chelate.
Sodium (Na)
Total Dissolved Salt (TDS) – Hardness
Sodium Adsorption Ratio (SAR)
Adjusted SAR pHc
pHc2
ORGANIC MATTER – Desired Level 3 – 5%
The organic matter measurement is an estimate of the amount of organic residue in the soil. In general, organic matter levels increase as soil gets darker in color. Organic matter is beneficial because it affects soil physical properties such as drainage and water retention. The organic matter content of the mineral soils is normally in a range of 1-8 %. Where practical, strive for a level of 3-5%; which is optimal or retention yet has properties of being permeable to move more water.
PPM (parts per million)
Results for the major and minor elements are reported in PPM. An acre of mineral soil 6-7 inches deep weighs approximately 2 million pounds. Therefore, to convert PPM to pounds per acre, multiply by 2.
CATION EXCHANGE CAPACIY (CEC)
CEC is a measurement of capacity of the soil to hold exchangeable cations. These include:
- Potassium (K)
- Magnesium (Mg)
- Calcium (Ca)
- Sodium (Na)
- Hydrogen (H) The CEC is largely on the amount and type of clay present and the organic matter content. In general, the higher the CEC, the higher the clay content of the soil.
- Sandy soils will have CEC’s <5
- Loamy soils CEC’s from 5-15
- Clay soils CEC’s>15
It is possible to increase the CEC in sandy profiles by increasing the amounts of organic matter.
Base Saturation
Base Saturation refers to the portion of the CEC occupied by K, Mg, Ca, and Na. Optimum potassium levels vary with the CEC. The following are some general ranges for CEC’s:
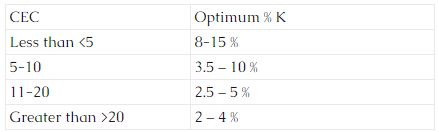
Magnesium levels are considered optimum when the base saturation level is 10 – 15% and calcium is at 65 – 75 %. Sodium levels should be <than 2 %.
NITRATE (NO3)
Nitrate-Nitrogen is the amount of available nitrogen present in the soil at the time it was analyzed in the laboratory. Because of its solubility, it can leach rapidly in various soil conditions. This mobility makes it difficult to predict how much nitrogen will be present throughout the growing season. However, it can be a useful tool for determining nitrogen utilization efficiencies at the end of the growing season.
To convert PPM into pounds/acre use the following formula:
Sample depth (in inches) x .333 = Conversion Factor (CF)
CF x NO3 PPM = NO3 pounds/acre
Example:
Soil test = 7 PPM NO3
Sample collection depth = 0 to 8 inches
8 x .333 = 2.66
7 PPM x 2.66 = 19 pounds/acre Nitrate-Nitrogen
Soils have the capacity to resist changes in pH, but there are instances where the water pH can cause changes. Both the soil and the water contain negatively and positively charged ions that influence the chemical composition and thus the pH of soil. Some soils are more resistant to change while other types can change rapidly if the water pH is significantly different from the soil matrix.
A soil’s ability to be influenced by the pH of the water is related to its texture. Soil particles that are smaller, like clays and clay loams, are more influenced than coarse, sandy soils. Fine-textured soils have a higher number of very small particles called colloids. These colloids are sites where positively charged ions are retained. The ability of a soil to retain these ions is called its cation exchange capacity (CEC). Ions in the soil solution are exchanged with ions on the colloidal particles. Negative ions in the soil solution have less influence on soil pH.
Desired Soil Results – Desired Values
pH 6
Hardness (ppm) 0 – 60
Conductivity (mmhos/cm) 0.75 – 2.25
Sodium Adsorption Ratio (SAR) 0 – 4
Adjust SAR 0 – 7
pHc 8.4
pHc2 8.4
TOTAL WATER APPLIED
PPM:
Calcium (Ca) 40 – 120
Magnesium (Mg) 6 – 24
Potassium (K) .5 – 10
Sodium (NA) < 40
Iron (Fe) 2 – 5
TOTAL CATIONS
Total Alkalinity (as CaCO3) 1 – 100
Carbonate (CO3) 0
Bicarbonate (HCO3) < 120
Hydroxide (OH) 0C
Chloride (CI) < 140
Sulfur (SO4) < 200
TOTAL ANIONS
Total Salt Concentration (TDS) < 640
Boron (B) .2 – .8
Cation/Anion Ratio
Bicarbonate Factor 18.75
How Water pH Can Overwhelm the Buffering Capacity of a Soil?
In normal rainwater and acid rain, there are excess hydrogen ions that can change soil pH by displacing calcium, aluminum and magnesium ions from the soil colloids.
The buffering capacity becomes overwhelmed when the positive ions attached to the colloids are exchanged for the hydrogen ion from the water source. The calcium, aluminum and magnesium ions migrate downward in the soil profile and the soil becomes acidic. High rainfall areas are more prone to this problem, and acid rain can speed up the process. In arid environments, soils are generally alkaline.
